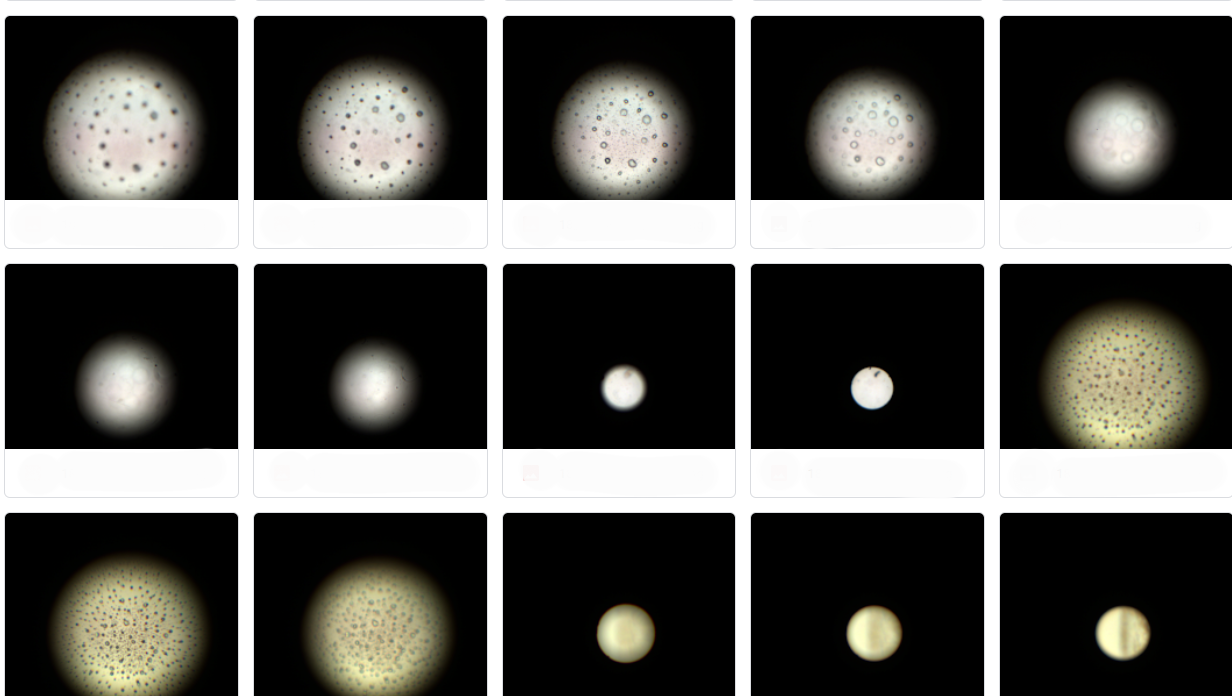
Automated Endoscopy Testing
Endoscope Tests
Years experience
Installed systems
Million images taken
Why testing is so important
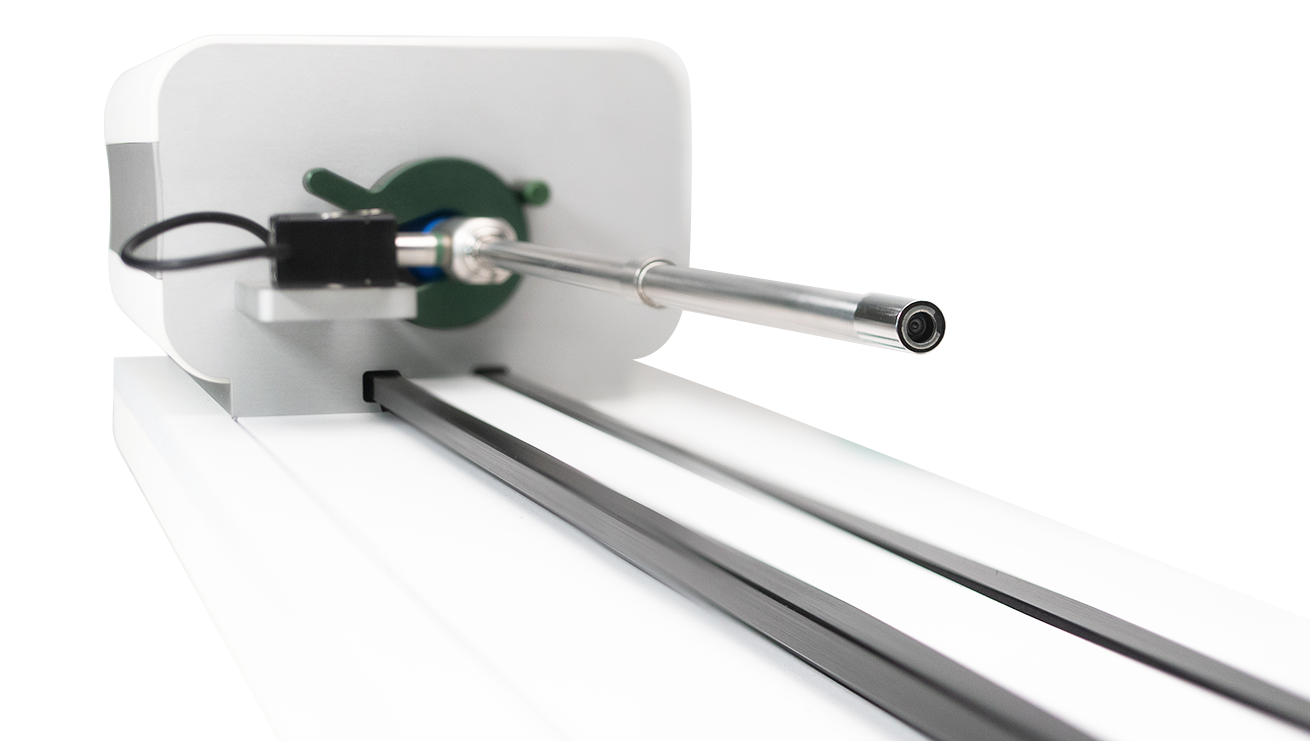
Regulatory Compliance and Automated Testing
With regulations like the EU MDR 2017/745 and ISO 17665 coming into effect in the coming years, hospitals must be able to prove their testing was done correctly.
Regulations also state that OEMs must provide hospitals with testing parameters. Because this is still lacking at the moment, Dovideq offers hospitals a solution that both tests their devices, as well as register all test results to a cloud platform.
What our customers say

Decontamination Services Manager
Jim Brown - NHS
''Although money is tight within the NHS, this equipment helps us to ensure a consistent service, improve productivity and enhance patient safety. ''

Medical Technology
Ber van Zon - Maastricht UMC
''ScopeControl secures our brand-agnostic testing of endoscopes, because its automated testing and reporting, increases patient safety and lowers overall endoscope costs bij 15%''

Head of medical instruments and control
Guido Kortleven - Ijsselland Ziekenhuis
''The first objective measuring method, Results are stored automatically, helping to secure procedures according to the MIC committee (minimal invasive surgery) guidelines and the European MDR. It reduces costs and is the best tool to avoid defective endoscopes from entering the operating theatre''
.png?width=225&height=225&name=images%20(1).png)
Head of CSSD
Matthias Wolf - Alb Fils Klinikum
''The biggest advantage is that we've saved a lot of money in a very short time''
Our Latest Blog Posts
- Dovideq Medical Systems
- August 14, 2025
- Dovideq Medical Systems
- July 4, 2025